Targeting Glioblastoma Through Metabolic Intervention
Glioblastoma (GBM) is one of the most aggressive and treatment-resistant forms of brain cancer, posing significant challenges for patients and healthcare providers alike. Traditional treatment approaches, including surgery, radiation, and chemotherapy, offer limited success in improving long-term survival. However, a growing body of research is now focusing on metabolic therapies, particularly the ketogenic diet, as a promising adjunctive treatment strategy. The ketogenic diet influences metabolic pathways in glioblastoma by targeting the altered energy metabolism of cancer cells, known as the Warburg Effect.
The Warburg Effect and Cancer Metabolism
First described by Otto Warburg a century ago, the Warburg Effect refers to the preference of cancer cells for glycolysis over oxidative phosphorylation, even in the presence of sufficient oxygen. This metabolic reprogramming allows cancer cells to rapidly generate energy and biomass to support their uncontrolled growth. Glioblastoma cells, in particular, exhibit a high dependency on glucose and glutamine as primary energy sources.
By restricting glucose availability and promoting the utilization of ketone bodies as an alternative fuel, the ketogenic diet aims to exploit the metabolic vulnerabilities of glioblastoma cells. Unlike cancer cells, normal brain cells can efficiently metabolize ketones for energy, which provides a unique therapeutic window to selectively target malignant cells while sparing healthy tissue.
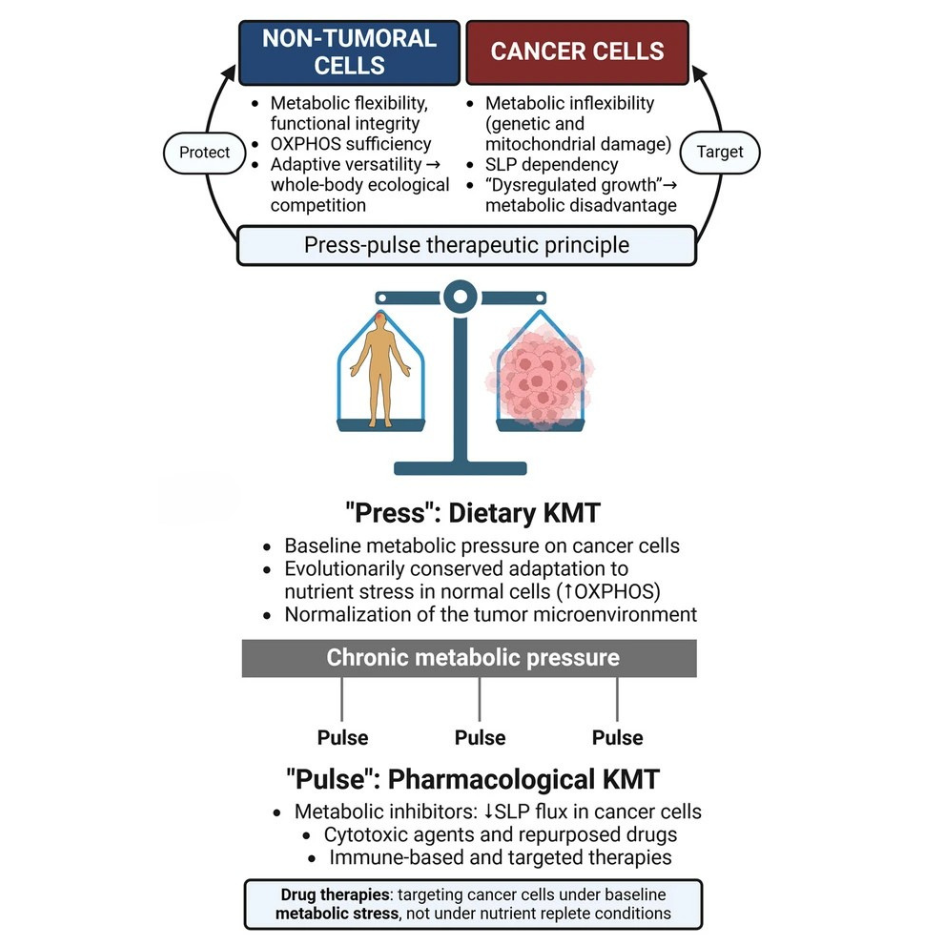
Simplified diagram of normal and cancer cell metabolism
Duraj et al. BMC Medicine (2024) 22:578
- All living cells need energy to work, and they get most of this energy in the form of ATP (a molecule that stores energy). Normal cells produce most of their ATP through a detailed, multi-step process called oxidative phosphorylation (OXPHOS), which happens in the mitochondria (the cell’s energy factories).
- Cancer cells instead, have damaged or unusual mitochondria, which makes this process less efficient. Cancer cells also have higher needs for making new molecules (biosynthesis) and managing harmful byproducts (redox balance). To make up for their less efficient oxidative phosphorylation, cancer cells boost other ways of producing energy, both inside and outside the mitochondria.
The Therapeutic Potential of Ketogenic Metabolic Therapy
The recent publication of the “Clinical Research Framework Proposal for Ketogenic Metabolic Therapy in Glioblastoma” in BMC Medicine commemorates the centennial of the Warburg Effect with a groundbreaking proposal. Led by Dr. Thomas N. Seyfried and a team of international experts, the study outlines how ketogenic metabolic therapy (KMT) can offer a novel, less toxic approach to glioblastoma treatment.
KMT works by shifting the body’s metabolism towards fat utilization and ketone production, effectively reducing the availability of glucose and glutamine to tumor cells. This metabolic shift forces glioblastoma cells to rely on substrate-level phosphorylation (SLP) via glycolysis, which is less efficient and ultimately hampers tumor growth and proliferation.
A Structured Clinical Research Framework
The proposed framework for investigating KMT in glioblastoma patients consists of several key components:
- Patient Selection Criteria: Identifying candidates based on clinical and molecular characteristics to optimize treatment outcomes.
- Study Design: Implementing randomized controlled trials with well-defined control groups to ensure rigorous evaluation of therapeutic efficacy.
- Outcome Measures: Focusing on critical endpoints such as overall survival, progression-free survival, and quality of life improvements.
- Safety Monitoring: Establishing protocols to manage potential side effects and ensure patient safety throughout the treatment.
The authors highlight the need for a multidisciplinary approach and standardized methodologies to enhance the reproducibility and reliability of research findings. Personalised treatment strategies, taking into account individual metabolic profiles and tumor biology, are also emphasized to maximize therapeutic success.
Broader Implications Beyond Glioblastoma
The insights gained from this research have implications that extend beyond glioblastoma. According to the metabolic theory of cancer, mitochondrial dysfunction and respiratory insufficiency are common features across various cancer types. The KMT framework can therefore serve as a versatile model for exploring metabolic therapies in other malignancies, offering potential benefits in a range of cancers where conventional treatments remain inadequate.
Conclusion
The ketogenic diet presents a promising avenue for targeting glioblastoma by addressing its metabolic vulnerabilities. The structured clinical research framework proposed by Dr. Seyfried and his team lays a solid foundation for future trials, with the potential to revolutionize glioblastoma treatment and improve patient outcomes. This approach aligns with The Dr. Coy Principle, which emphasises carbohydrate management to inhibit cancer cell fermentation pathways. By limiting glucose intake and incorporating specific sugars like galactose and mannose, The Dr. Coy’s principle aims to successfully shift cancer cell metabolism from fermentation to combustion, thereby reducing their growth and enhancing the effectiveness of conventional therapies.
As research progresses, integrating these metabolic strategies may become an integral part of comprehensive cancer care, providing new hope for those battling this devastating disease.
References
Commemorating the Centennial of the Warburg Effect with a Visionary Proposal


